
[Curator’s note: The following material quotes and paraphrases extensively from articles posted by the Nobel Prize Committee, the Princeton Alumni Weekly. Sources below for details.]
The Nobel Prize in Chemistry: 1996
The Nobel Prize in Chemistry 1996 was awarded jointly to Robert F. Curl Jr., Sir Harold W. Kroto and Richard E. Smalley "for their discovery of fullerenes."
Work
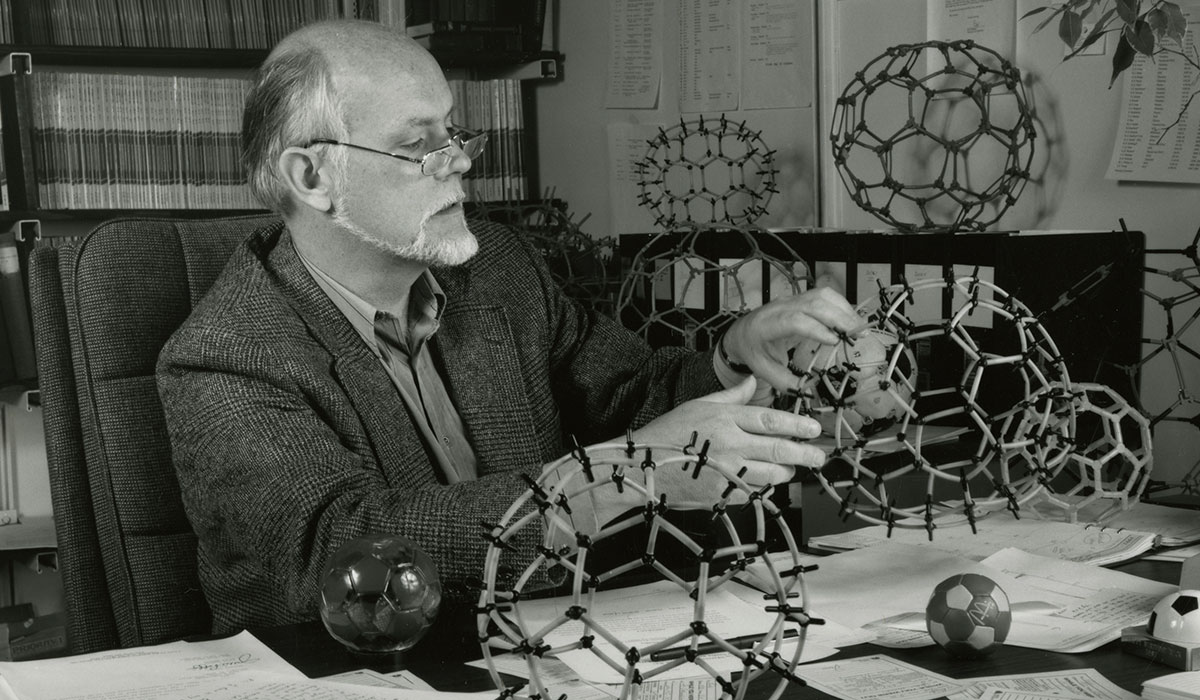
Carbon is an element that can assume a number of different forms. In nature, for example, graphite and diamonds appear. In 1985 Richard Smalley, Robert Curl, and Harold Kroto irradiated a surface of graphite with laser pulses so that carbon gas was formed. When the carbon gas condensed, previously unknown structures with 60 and 70 carbon atoms were formed. The most common structure had 60 carbon atoms arranged in a sphere with five and six edges. The structures were called fullerenes in honor of architect Buckminster Fuller, who worked with this geometric shape.
Early life
Richard Smalley was born in Akron, Ohio. He was the youngest of four children and when he was three his family moved to Kansas City.
The principal impetus for Richard’s entering a career in science was the successful launching of Sputnik in 1957, and the then current belief that science and technology was going to be where the action was in the coming decades. He suddenly became very serious with his education at the beginning of his junior year in the fall of 1959.
“This happened to be the year when I began to study chemistry for the first time. My mother’s youngest sibling, Dr. Sara Jane Rhoads, was one of the first women in the United States to ever reach the rank of full Professor of Chemistry. After earning her Ph.D. in 1949 with William von Eggers Doering, who was then at Columbia University, she devoted her life to teaching and research in the Department of Chemistry of the University of Wyoming. She received the Garvan Medal of the American Chemical Society in 1982 for her contributions to physical organic chemistry, particularly in the study of the Cope and Claisen rearrangements. She was the only scientist in our extended family and was one of the brightest and, in general, one of the most impressive human beings I have ever met. She was my hero. I used to call her, lovingly, ‘The Colossus of Rhoads’. Her example was a major factor that led me to go into chemistry, rather than physics or engineering. One of the most enjoyable memories of my early life was the summer (1961) I spent working in her organic chemistry laboratory at the University of Wyoming. It was at her suggestion that I decided to attend Hope College that fall in Holland, Michigan. Hope had then one of the finest undergraduate programs in chemistry in the United States.”
An education at Hope College and Shell Chemical Company
Smalley spent two years at Hope College but decided to transfer to the University of Michigan in Ann Arbor after his favorite professor, Dr. J. Harvey Kleinheksel, died of a heart attack, and the organic chemistry professor with whom he had hoped to do research, Dr. Gerrit Van Zyl, announced his retirement.
By the time of my graduation in 1965, the job market for scientists in the United States was at an all-time high, and even chemistry graduates with just a BS degree were in great demand. Rather than proceeding directly to graduate school, he decided to take a job in the chemical industry to buy a bit of time to see what he really wanted to do in science, and to live a little in the “real” world.
In the fall of 1965, he began work full time in Woodbury, New Jersey at a large polypropylene manufacturing plant owned by the Shell Chemical Company. Smalley began as a chemist working in the quality control laboratory for the plant, a 24 hour a day operation that in the mid 60’s was quite a wonderland of high technology. His first boss was a chemist named Donald S. Brath. He taught his young professionals that “chemists can do anything” and the time spent working for him was a broadening experience.
Although he found his work at Shell highly enjoyable, he realized it was time to get on to graduate school, so he began to study seriously and to send out applications. At the time, Smalley was most interested in quantum chemistry and received several offers for graduate assistantships in excellent schools. He was close to accepting an offer from the Theoretical Chemistry Institute at the University of Wisconsin when the automatic graduate student deferments from the draft into the US military were eliminated.
During that time, he met Judith Grace Sampieri and they were married on May 4 of 1968. Soon thereafter, he decided to reapply to graduate school. Since Judy’s family lived in New Jersey, he decided to apply to Princeton University, and was accepted. In the late Fall 1968, he was reclassified 1A for the draft and reported to the processing center in Newark for his physical. However, within a week, his wife was pregnant, and within just a few more weeks his draft board reclassified him, so that he would not be drafted.
Princeton and the University of Chicago
In the Fall of 1969, he moved to Princeton to begin studies and research for the Ph.D. in the Department of Chemistry. He was in the first group of graduate students to work with Elliot R. Bernstein who was just starting as an Assistant Professor at Princeton, after having spent a few years postdoctoral work at the University of Chicago with Clyde A. Hutchison III, following doctoral training with G. Wilse Robinson at CalTech.
Elliot’s research at the time involved detailed optical and microwave spectral probes of pure and mixed molecular single crystals cooled in liquid helium. He knew nothing about it at the time he joined the group. His research project was the detailed study of 1,3,5-triazine, a heterocyclic benzene analog that they expected would provide a poignant testing ground for theories of the Jahn Teller effect. In the end he found that the crystal field surrounding each molecule was insufficiently symmetrical to provide the tests he originally sought, but much was learned. Most importantly from his standpoint, he learned from Elliot Bernstein a penetrating, intense style of research that he had never known before, and a great deal about the chemical physics of condensed phase and molecular systems.
In the summer of 1973, he moved to the south side of Chicago so he could begin a postdoctoral period with Donald H. Levy at the University of Chicago. Levy had studied gas-phase magnetic resonance with Alan Carrington and had been doing some of the most impressive research anywhere in the world with microwave/optical double resonance and the Hanle effect on NO2 and other open-shell small molecules.
These were the earliest days when tunable dye lasers were beginning to transform molecular spectroscopy, and Levy’s group was in the lead. The optical spectrum of NO2 was the most troublesome problem for molecular spectroscopists. Even though it had only three atoms, the visible spectrum had far more structure than anyone could understand. But since NO2 was readily available and it displayed an extensive absorption spectrum just where the new lasers could readily operate (500-640 nm), it was a favorite object for study.
“Don Levy and one of his students, Richard Solarz, had made some major advances with NO2 earlier that summer, so after I arrived in Chicago, I began to consider what I could do next. My biggest problem was that my training at Princeton had been in condensed matter spectroscopy, and the ultrahigh resolution gas-phase spectral techniques being used by the Levy group were going to take months to understand. The detailed physics of rotating polyatomic molecules with spin is extremely complex. I was familiar only with the physics of molecules frozen still in a crystal lattice near absolute zero.”
When he arrived in Chicago, Don Levy was in Germany for a several month-long visit, so he had an opportunity to do some extended reading and to prepare for the final oral exam for the Ph.D. degree back in Princeton. At that time in the Chemistry Department at Princeton, the final oral exam consisted of a defense of three original research proposals. Smalley spent many hours in the Univ. Chicago chemistry department library reading recent journal articles, searching for possible topics for these research proposals.
He had read a new paper by Yuan Lee and Stuart Rice on the crossed beam reaction of fluorine with benzene (J. Chem. Phys. 59, 1427 (1973)] in one of Yuan’s “universal” molecular beam apparatuses. It was the sort of experiment that was to lead to Yuan Lee sharing the Nobel Prize in 1986 with John Polanyi and Dudley Herschbach.
Smalley was deeply struck by a passage in the paper which said that the supersonic expansion used to make the benzene molecular beam was strong enough to cool out essentially all rotational degrees of freedom. That was just what he needed. Since he didn’t understand rotating molecules yet, perhaps he could just stop them from rotating in the first place!”
He received his Ph.D. from Princeton University in 1973 after completing a doctoral dissertation, titled "The lower electronic states of 1,3,5 (sym)-triazine", under the supervision of Elliot R. Bernstein. He did postdoctoral work at the University of Chicago from 1973 to 1976, with Donald Levy and Lennard Wharton where he was a pioneer in the development of the supersonic beam laser spectroscopy.
Professor of Chemistry at Rice University
At Rice University, where he began his career in 1976, he established himself as a highly creative scientist who opened new fields of research about every two years. Having conceived a new approach to some phenomenon, he constructed the necessary apparatus, demonstrated the potential of his method, and moved forward.
In 1985 Smalley and two colleagues discovered a geodesic dome-like form of carbon called buckminsterfullerenes, for which they won the Nobel Prize for chemistry in 1996. With the subsequent isolation of related carbon nanotubes, Smalley focused on placing the new field of nanotechnology on firm ground. Even as he battled cancer, he worked tirelessly to convince Congress and the world that nanotube technology held tremendous promise for advances in medicine and energy.
Nobel Prize-winning chemist Richard Errett Smalley died of leukemia Oct. 28, 2005, in Houston, Texas.
SOURCES
• The Nobel Prize in Chemistry 1996. NobelPrize.org. Nobel Prize Outreach AB 2022
• Les Prix Nobel. The Nobel Prizes 1996 , Editor Tore Frängsmyr, Nobel Foundation, Stockholm, 1997. This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
• Source: PAW: Richard E. Smalley Memorial
OTHER SOURCES
• American Institute of Physics
• Nature

